Not just water: Understanding the main ingredient of pools

One thing that swimming pools throughout the entire world have in common is their main ingredient: water. It might seem like a trite opening for this article. While water’s presence in a pool is obvious, it is surprising how often it is taken for granted among many service technicians and operators. Water is the most vital chemical in a pool. Yes, water is a chemical. Like any other chemical used for treatment, it reacts based on its local composition and environment. Does the industry fully understand the makeup of the essential chemical introduced when a pool is initially filled and each time it is topped up? Most damage to pool surfaces during start-up or maintenance results from a lack of knowledge about the water’s chemical balance.
Characteristics of water
Water is regarded as an inorganic substance. However, it can sometimes behave quite like a living entity in the way it reacts, responds, and changes. Three traits of water make it special. First, water is amphoteric, meaning it has the ability to seek its own balance. Pure water, for instance, has a pH of 7.0, which is considered neutral on the scientific scale. In this case, water remains neutral because it can gain a proton to form a hydronium ion (H3O+), and donate a proton to form a hydroxide ion (OH–). Water does this in equilibrium through a process known as auto-ionization. Two H2O molecules in pure water react to produce equal amounts of acid and base. When water reacts with certain bases, it produces acids, and when it reacts with acids, it produces bases. Unless chemically altered, water left on its own will react based on the environment it is in.
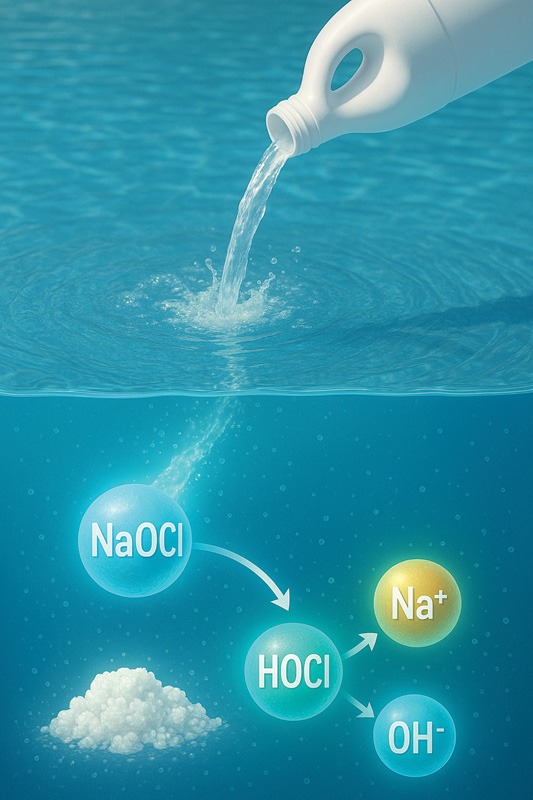
The second characteristic of water is its ability to break down compounds through a process known as hydrolysis. Think of this term as a water knife. Meaning that water reacts with various chemical compounds, it cuts the compounds into differing parts. An example of hydrolysis is the reaction between water and chlorine sanitizer. All forms of chlorine used in pools are compounds. One example is liquid chlorine, also known as sodium hypochlorite (NaOCl). The hydrolysis reaction begins as soon as liquid chlorine comes into contact with water. Immediately, the water reacts and breaks down the sodium hypochlorite into hypochlorous acid (HOCl), sodium (Na+), and OH–. HOCl is the killing agent of chlorine with a 99 per cent power. Hydrolysis goes further as the HOCl dissociates into a hydrogen ion, H+, and a hypochlorite ion, OCl–. This is a simple example illustrating that the hydrolysis of water produces free chlorine (FC) from a chlorine compound.
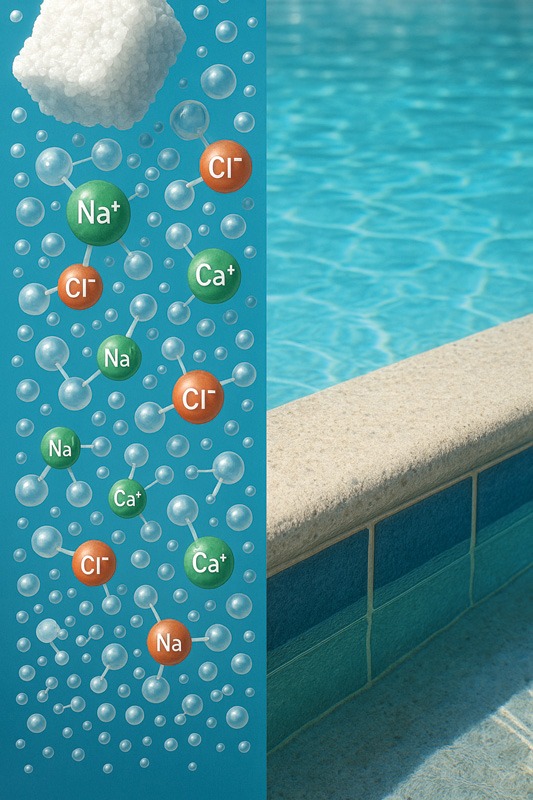
This leads to the third characteristic of water, which is its role as a universal solvent. Essentially, this means water can dissolve solid materials such as cement or salt. When dissolving salt (NaCl), water, a polar molecule, interacts with the salt, another polar molecule. Adding salt to water triggers a hydrolysis reaction that begins to separate the positively charged sodium ions (Na+) from the negatively charged chloride ions (Cl–). As the ions break apart, the polarity of water molecules comes into play, with the positive hydrogen atoms orienting toward the negative chloride ions and the negative oxygen atoms toward the positive sodium ions. Consequently, water molecules surround the salt ions, keeping them dissolved and separated. Notably, many chemicals used in pools are derived from salt. For example, chlorine is produced from salt, and chemicals such as sodium carbonate and sodium bicarbonate are used to balance the water’s pH level. A prime example of water as a universal solvent is the Grand Canyon, where over several million years, between 416,800 and 1,250,500 km3 (100,000 and 300,000 mi3) of rock and debris were eroded by the Colorado River and its tributaries. In a pool, the combination of water’s properties and gentle circulation can, over time, erode cementitious aggregate.
Quick reference: Treat water as a chemical
- Water is the most vital chemical in a pool.
- It reacts based on source makeup and environment.
- Most start-up damage occurs due to a poor understanding of water balance.
- Water must not be treated as a passive filler.
- Always assess source water before filling any pool.
- Unexamined water can cause plaster etching, scaling, and surface degradation.
Regional differences and start-up
Understanding that water is a chemical and that it reacts differently depending on regional locations is essential for pool startup. Some parts of the world have more surface water, such as rivers and lakes. This water is relatively new and has not eroded or accumulated many minerals. In these areas, the source water used to fill pools will be soft. As a chemical, soft water can be harmful to pool surfaces and metallic equipment. It acts as an acid seeking a base, because it is low in mineral calcium (Ca). The water will try to extract calcium from the plaster to balance itself, causing etching and serious damage to the surface. Vinyl liners and fibreglass surfaces can also degrade and be damaged by the force of
soft water.
Quick reference: Water behaviour essentials
Amphoteric (seeks balance)
- Can behave as both an acid and a base.
- Neutral pH of pure water: 7.0
- Forms hydronium ion (H3O+) and hydroxide ion (OH–) through auto-ionization.
Hydrolysis (water knife)
- Breaks compounds into parts
- Sodium hypochlorite (NaOCl) breaks into:
- Hypochlorous acid (HOCl)
- Sodium ions (Na+)
- Hydroxide ions (OH–)
- Produces free chlorine (FC)
Universal solvent
- Dissolves salt, cement, minerals
- Separates Na+ and Cl– ions
- Contributes to long-term erosion of cementitious surfaces
Hard water is water that has been underground for either hundreds of years or just a few weeks. During that time, it absorbs minerals from the earth, including calcium and magnesium (Mg). This causes the water to become oversaturated, leading to the formation of scale on pool surfaces and equipment. Water with high calcium levels in warmer areas can be particularly challenging because it promotes the formation of calcium carbonate (CaCO3) scale.
The lesson here again is to understand that the essential chemical water will vary in consistency depending on its source. When filling a new pool, it is not the time to test or adjust the water after it is filled. A proper pool start-up begins by knowing the source water before turning on the hose, that is, before the pool is filled. Source water should be tested for pH, alkalinity, calcium, total dissolved solids (TDS), chlorine level, source metals, nitrates (NO–3), phosphates, and temperature. Based on these results, treatment can be applied as the pool fills to reduce the risk of finish damage. The temperature of the fill water is a concern as colder water is more corrosive, and warmer water is more reactive. Cold water tends to dissolve calcium, while warmer water causes calcium to precipitate as a solid. Temperature also affects the Langelier Saturation Index (LSI) test. Another important factor is TDS. Higher TDS levels create a more corrosive environment in the pool. Conversely, low TDS might also be problematic, as it indicates a lack of minerals, which could cause the water to seek minerals from pool materials if it is not chemically balanced.

Tame the beast
Water is a highly unpredictable, reactive, and mysterious element. Before it enters the pool, it needs to be examined, defined, and understood. Taking water for granted and failing to treat it like any other chemical can lead to many damaging situations, from plaster etching to metal stains. The best advice is to tame the beast before it is set free. That means testing and understanding the source water before it goes into the pool.

Quick reference:
Pre-fill source water testing
Test “before” filling:
- pH
- Alkalinity
- Calcium
- Total dissolved solids (TDS)
- Metals
- Nitrates (NO–3)
- Phosphates
- Chlorine level
- Temperature
Soft water indicators and risks
- Low calcium
- Extracts calcium from plaster
- Causes etching and surface damage
- Can degrade vinyl and fibreglass
Hard water indicators and risks
- High calcium and magnesium
- Promotes scale formation
- Warm conditions increase calcium carbonate (CaCO3) deposits
Temperature impacts
- Cold water dissolves calcium (more corrosive)
- Warm water precipitates calcium (solid formation)
- Affects Langelier Saturation Index (LSI)

Bringing it all together
In summary, the significance of water as the core chemical in every swimming pool cannot be overstated. Its unique characteristics, being amphoteric, acting as a universal solvent, and breaking down compounds through hydrolysis, make it both essential and somewhat unpredictable. Regional differences in water composition can significantly impact pool surfaces and equipment, underscoring the importance of a thorough understanding before filling the pool. By respecting water’s reactive nature and testing source water for key parameters, pool operators can prevent costly damage and maintain a safe, balanced environment. Ultimately, treating water as the dynamic chemical it is remains vital for successful pool maintenance and longevity.
Author
Terry Arko is a product training and content manager for HASA Pool Inc., a manufacturer and distributor of pool and spa water treatment products in Saugus, Calif. He has more than 40 years of experience in the pool and spa/hot tub industry, working in service, repair, retail sales, chemical manufacturing, technical service, commercial sales, and product development. He has written over 100 published articles on water chemistry and has been an instructor of water chemistry courses for more than 25 years. Arko serves as a voting member on the Recreational Water & Air Quality Committee (RWAQC) board. He is a Commercial Pool Operator (CPO) course instructor, a Pool Chemistry Certified Residential course teacher for the Pool Chemistry Training Institute (PCTI), and a Pool & Spa Marketing Editorial Advisory Committee member. Arko can be reached at terryarko@hasapool.com.






